TTR Destabilization:
The Source of ATTR‑CM
Discover the causes, symptoms, outcomes, and diagnostic methods of this relentlessly progressive heart disease.1
ATTR-CM is also known as ATTR cardiomyopathy, or ATTR cardiac amyloidosis (ATTR-CA).
This site is intended for US Healthcare Professionals only.



What Is TTR?
TTR, also known as prealbumin, plays a role in processes vital to human health2,3:
- Highly conserved transport protein produced in the liver
- Distributes thyroxine and vitamin A throughout the body
- Supports memory, neuroprotection, and cognitive function
Click play to learn more about TTR.
TTR Destabilization in ATTR‑CM
TTR destabilization is the root cause of ATTR‑CM4,5
Factors such as aging or TTR gene variants can trigger a cascade of structural changes that destabilize the configuration of circulating TTR tetramers.4 These changes may cause the tetramers to break apart and misfold.5 Misfolded TTR proteins can aggregate into toxic amyloid fibrils that cause signs and symptoms indicative of transthyretin amyloidosis.5,6
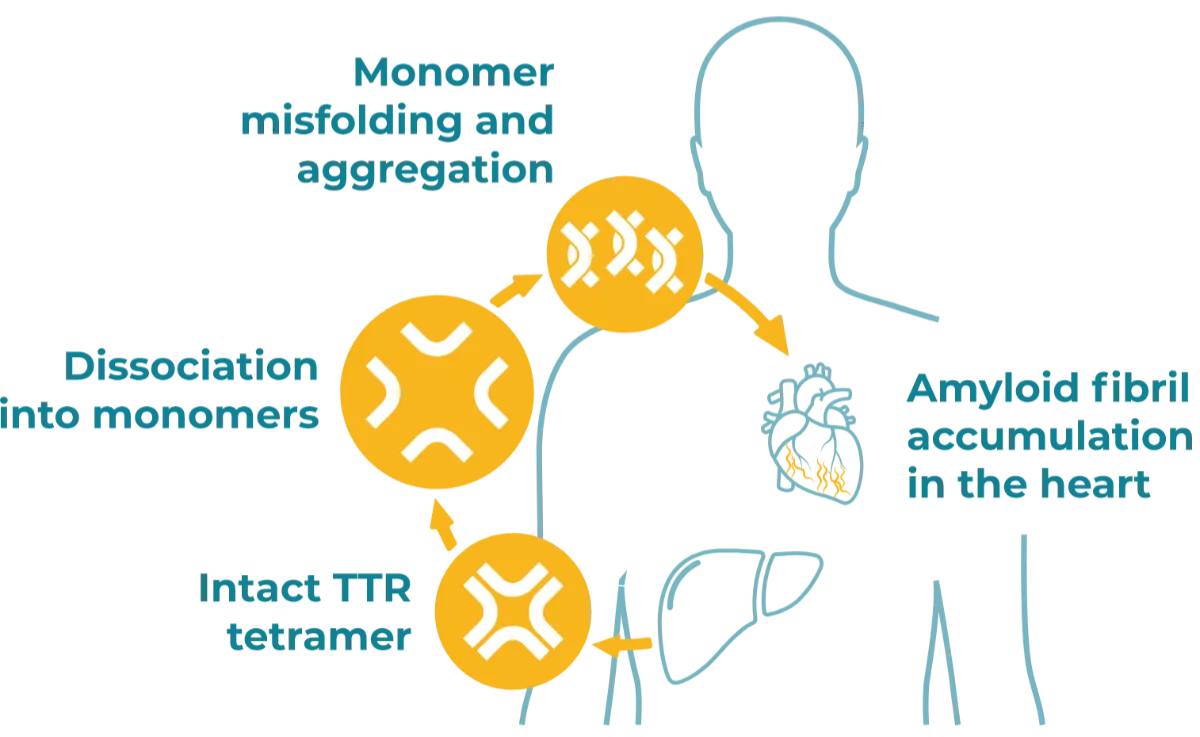
*Amyloid fibrils can also accumulate in areas of the body outside of the heart.6,7
TTR Stability Impacts Disease Severity
Greater TTR destabilization is correlated with earlier disease onset and increased disease severity7
TTR gene variants strongly influence the rate of TTR tetramer dissociation, which is required for amyloid formation.7 Most known variants destabilize the tetramer, increasing the amyloidogenic potential of TTR and leading to poor clinical outcomes.7 However, variants shown to stabilize the tetramer have also been identified.6 These variants prevent amyloidogenesis by dramatically slowing the rate of tetramer dissociation and have been associated with disease protection.6,7


Relative tetramer stability based on denaturation midpoints measured via an in vitro dissociation/unfolding assay.7
The most beneficial TTR gene variant identified to date is T119M, which has been shown to confer protection against the development of ATTR even when coinherited with variants associated with poor outcomes7
Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16-26. 2. Liz MA, Coelho T, Bellotti V, Fernandez-Arias MI, Mallaina P, Obici L. A narrative review of the role of transthyretin in health and disease. Neurol Ther. 2020;9(2):395-402. 3. Vieira M, Saraiva MJ. Transthyretin: a multifaceted protein. Biomol Concepts. 2014;5(1):45-54. 4. Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142(1):e7-e22. 5. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872-2891. 6. Hornstrup LS, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Genetic stabilization of transthyretin, cerebrovascular disease, and life expectancy. Arterioscler Thromb Vasc Biol. 2013;33(6):1441-1447. 7. Hammarström P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci USA. 2002;99(suppl 4):16427-16432. 8. Morfino P, Aimo A, Vergaro G, et al. Transthyretin stabilizers and seeding inhibitors as therapies for amyloid transthyretin cardiomyopathy. Pharmaceutics. 2023;15(4):1129.
The information provided on this site is intended for use by healthcare professionals practicing in the United States.
By clicking ENTER you confirm you are a healthcare professional practicing in the United States.
Thank you for visiting TTRMatters.com
If you are a healthcare provider and want to learn more about an ATTR-CM treatment option, click "Continue."
To remain on TTRMatters.com, click "Stay."
